|
Choice of Therapy
We are currently in an era in which there are several options to consider for this patient, which was not the case about 10 years ago. These options include intensive chemotherapy induction or lower intensity therapy with hypomethylating agent backbone. In this particular patient, being in her early 70s but in good health, she could also be considered for a future and potentially curative allogeneic stem cell transplant. Thus, in terms of the treatment options for this patient, we would, as mentioned, consider her a candidate for intensive therapy. I believe that given her excellent performance status and lack of comorbidities, and potential candidacy for bone marrow transplant, that induction chemotherapy would be very reasonable. What I would choose in this patient's case would be CPX-351 or liposomal cytarabine and daunorubicin. CPX-351 is a liposomal encapsulated formulation of both cytarabine and daunorubicin in a fixed molar concentration ratio of 5:1.
Supporting data for CPX-351
CPX-351 has been studied over the past several years. The definitive trial was conducted a few years ago, in which patients with secondary AML (sAML) were randomly assigned to receive either CPX-351 or standard induction chemotherapy with daunorubicin and cytarabine, or 7+3. Results of this study indicated that survival was improved with CPX-351 vs 7+3 in this patient population, and the hazard ratio of 0.69 indicates a decrease in the risk of death over the course of the study by about 31% in favor of CPX-351 (Figure 1).1 In addition, in this particular trial, the remission rates were significantly higher with CPX-351 vs 7+3, with overall response rates of about 48% in the CPX arm vs 33% in the 7+3 arm.
Figure 1
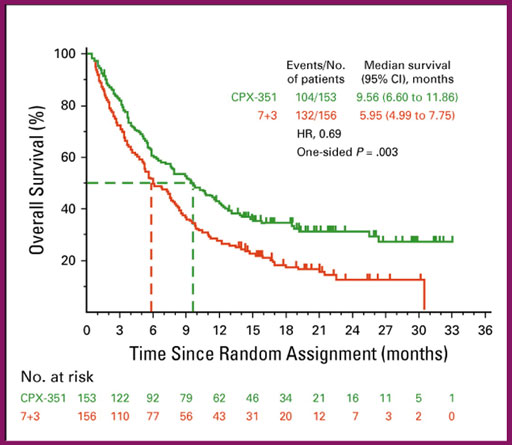
Figure 1. Overall survival results of CPX-351 vs 7+3 in patients with newly diagnosed secondary acute myeloid leukemia.1
Findings from a landmark analysis of overall survival from the time of stem cell transplant indicated that patients who received CPX-351 lived longer after transplant than patients who received 7+3 as their initial induction therapy (Figure 2).2 Taken together, these findings indicated that CPX-351 is an effective therapy and better than traditional induction chemotherapy for older patients with newly diagnosed secondary or therapy-related AML. Based upon this evidence and our patient's characteristics, I believe that CPX-351 would be the most appropriate therapy for such a patient.
Figure 2
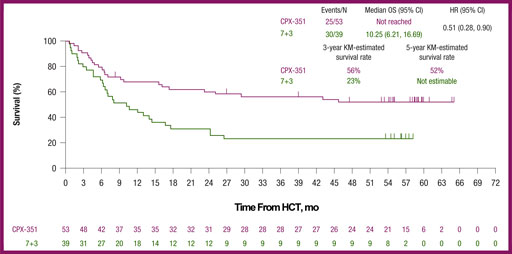
Figure 2. Five-year overall survival data from the time of HCT of CPX-351 vs 7+3 in patients with newly diagnosed secondary acute myeloid leukemia.2
Safety Profile of CPX-351
Regarding adverse events or toxicities that we can expect with CPX-351, it is important to recognize that CPX-351 is, of course, considered intensive induction chemotherapy and should be managed as such. The main toxicities that we think about with such a regimen would mainly be related to neutropenia and infection.1 These are not unique to CPX-351 but are something we must need to pay close attention to in our patients on this particular therapy.
In addition, it is important to recognize that the hematologic toxicities of CPX-351 are more pronounced than traditional induction chemotherapy with 7+3; specifically, the duration of neutropenia and thrombocytopenia is approximately 7 to 10 days longer with CPX-351 compared with 7+3 (Table 1).5 This is important because it can potentially leave patients at higher risk for infection over a longer period of time, and it is therefore important to be very judicious about when to begin to back off antibiotics and to monitor infections very closely.
Table 1. Prolonged Time to Recovery of Cytopenias Associated with CPX-351 vs 7+3.5
Measure, n (%) |
Induction |
Consolidation |
||
CPX-351 (n = 58) |
7+3 (n = 34) |
CPX-351 (n = 48) |
5+2 (n = 32) |
|
Prolonged thrombocytopenia |
16 (28) |
4 (12) |
12 (25) |
5 (16) |
Prolonged neutropenia |
10 (17) |
1 (3) |
5 (10) |
1 (3) |
The 7+3 control cohort received an initial induction regimen of cytarabine 100 mg/m2/d administered by 7-day continuous infusion with daunorubicin 60 mg/m2 on days 1 to 3. The second induction course and postremission consolidation courses consisted of cytarabine 100 mg/m2/d by 5-day continuous infusion with daunorubicin 60 mg/m2 on days 1 and 2.
The delayed recovery of blood counts is an important distinguishing feature of the toxicity profile of CPX-351 and it can be quite prolonged. It is not unusual to see patients go for sometimes several weeks before full count recovery occurs. This should be taken into context with respect to careful monitoring of patients and periodic bone marrow assessments to look for evidence of any residual leukemia that could be contributing to the persistence of cytopenias. However, it is important that physicians who use this CPX-351 recognize that there is the possibility of a significantly delayed count recovery that can still occur in the setting of an evolving remission. The message really is to try to remain patient while monitoring carefully, recognizing that there is overall a higher degree of hematologic toxicity in terms of duration.
In terms of other side effects or toxicities, there is little difference between standard chemotherapy and CPX-351.1 Overall, the usual risks of gastrointestinal-related toxicities and infections are rather similar. Notably, CPX-351 does not typically cause alopecia, which, although may seem like a minor point, is certainly something that may be quite important to patients as well.
It is important to recognize that the death rate, the early death rate, was not any worse or higher with CPX-351 compared to 7+3 in the phase 3 clinical trial, indicating that despite the fact that there were more prolonged cytopenias with CPX-351, that this did not translate into a higher risk of early death from any cause. In fact, the early death rates on the CPX-351 arm seem to be somewhat lower than the early death rate of the 7+3 arm, and this could be related to a lower chance of death related directly to leukemia progression if there is better overall response and disease control with CPX-351.
The Role of Venetoclax in Newly Diagnosed sAML
Some of the factors that go into the decision about treating a patient with a lower intensity regimen such as HMA/venetoclax vs CPX-351 in the newly diagnosed sAML setting are based upon patient-related factors such as age, comorbidities, functional status, and the suitability of a patient for an allogeneic transplant. So clearly, an older frail patient, who is not considered a transplant candidate, should preferentially receive HMA/venetoclax because it is not as intensive. However, it certainly has its risks and toxicities that have to be very carefully managed. Thus, I would not consider it light therapy, but it is less intensive than standard induction. Patients who have a more frail state or who have other medical comorbidities that might pose on acceptable toxicity risks from induction chemotherapy should be strongly considered for HMA/venetoclax.
The combination of hypomethylating agent (HMA) therapy, namely azacitidine, plus the BCL2 inhibitor venetoclax was recently demonstrated in a phase 3 randomized trial to have significant superiority in terms of overall survival compared with the use of single hypomethylating agent therapy, indicating that the combination of HMA plus venetoclax is also an appropriate therapy for older patients with newly diagnosed AML.3 However, that study was concentrated on older patients, in particular those aged over 75 years, and with additional comorbidities that precluded them from receiving or being considered candidates for intensive induction chemotherapy. Moreover, although HMA/venetoclax can be considered certainly as an option for older patients who are not candidates for or refuse induction chemotherapy, we have to recognize that these two strategies, CPX-351 and HMA/venetoclax, have not been compared head-to-head. Thus, we cannot speak to the superiority of one regimen vs the other. I think this is going to be one of the most important debates or dilemmas that we have over the next few years with respect to initial therapy for AML. We also need to recognize that the patient populations that were tested in the different trials, looking at CPX-351 and the HMA/venetoclax trial, were quite different. So, in the context of our particular patient, CPX-351 is perhaps the most appropriate therapy, given the fact that the patient wanted to be aggressive about her treatment and was also a legitimate bone marrow transplant candidate.
Choice of therapy for p53-mutated sAML
It is important to consider the molecular status of the patient's leukemia when it comes to a decision about the preferred induction regimen or the preferred first-line regimen. In patients with secondary AML in the phase 3 clinical trial with CPX-351, there seemed to be rather equivalent benefit of CPX-351 over 7+3, regardless of the patient molecular subtype, with the exception of the p53 mutations.4 This is a subgroup that does poorly regardless of the type of intensive induction chemotherapy, and it seems as though the CPX-351 does not overcome the adverse effect of the p53 mutation, such that it did not perform better than 7+3 in patients with initial p53 mutations at the time of diagnosis. Similarly, we know from the phase 3 data with HMA/venetoclax that patients with p53-mutant AML also did not have very good outcomes in the long run, in the sense that the survival was short as well as the disease-free survival, despite the fact that about half the patients with p53 mutations were able to achieve remission with the combination of HMA/venetoclax.3 Nonetheless, in a patient who is unlikely to achieve any type of a durable response because of the p53 mutation, in our practice, we would tend to not utilize induction with CPX-351 in such a patient because of the expected poor outcome and the higher intensity and higher toxicity profile of that regimen, which may not be worth it for the patient in the long run.
Ongoing Clinical Trials with CPX-351
CPX-351 is still in an early phase of development as it pertains to combination therapy. It has emerged as a standard of care option for patients with sAML who are considered candidates for induction chemotherapy, but that's not the end of the story. So certainly, when there is a new interactive drug, there is much interest in combining it with other agents. Currently, there are ongoing trials in which CPX-351 is being combined with other targeted agents such as venetoclax, or some of the other mutation-targeted therapies such as FLT3 inhibitors and IDH inhibitors. I always favor a clinical trial whenever possible because of the fact that CPX-351, although representing progress in the field, is still not sufficient in and of itself to be the definitive therapy as we wish it to be to induce a greater percentage of patients who are in long-term remission or cured state. Thus, it is always important to consider clinical trials and that applies really for any type of AML including sAML. But it is important to emphasize that although progress is being made, we still have a long way to go in order to achieve a higher long-term success rate in these patients.
References
- Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684-2692.
- Lancet JE, Uy GL, Newell LF, et al. Five-year final results of a phase 3 study of CPX-351 versus 7+3 in older adults with newly diagnosed high-risk/secondary AML. ASCO Annual Meeting. May 29-31, 2020. Poster 283. Available at https://vyxeos.eu/wp-content/uploads/2020/05/ASCO_2020_Study_301_5-year_Final_Results_Poster_FINAL.pdf Accessed January 18, 2021.
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383:617-629.
- Lindsley RC, Gibson CJ, Murdock M, et al. Genetic characteristics and outcomes by mutation status in a phase 3 study of CPX-351 versus 7+3 in older adults with newly diagnosed, high-risk/secondary acute myeloid leukemia (AML). Blood. 2019:134(Supplement_1):15.
- VYXEOS™ (daunorubicin and cytarabine injection), solution for intravenous use [package insert]. Palo Alto, CA; Jazz Pharmaceuticals, Inc.; 2019.
This activity is supported by educational grants from Actinium Pharmaceuticals, Bristol-Myers Squibb, and Jazz Pharmaceuticals.
